Blog
Understanding the Different Types of Vitiligo

Vitiligo is one of the most misunderstood autoimmune skin disorders, affecting an estimated 2.8 million people in the United States. Even so, few people understand what vitiligo is, ...
Learn MoreWhy You Should Consider Participating in a Dermatology Clinical Trial
Have you ever considered participating in a dermatology clinical trial? Clinical trials are important for developing new, effective treatments for a variety of skin conditions from acne to ...
Learn More5 Most Common Causes of Psoriasis Flare-Ups

One of the most common skin disorders, psoriasis, is an autoimmune response in which skin cells multiply at an accelerated rate. This leads to the formation of scaly, ...
Learn More5 Health Checks to Get Before Your Deductible Resets

As the year draws to a close, now is the best time to take advantage of your health insurance deductible. While preventive screenings are free under the Affordable ...
Learn MoreHealthy Skin Month: 5 Essential Skincare Tips Every Dermatologist Recommends

November is National Healthy Skin Month, so we are sharing our top suggestions to care for your skin every day. Aside from helping minimize the signs of aging, these skincare ...
Learn MoreI Found a New Mole. Should I Get it Checked?

Despite being largely preventable, cutaneous melanoma, a type of skin cancer, is the most serious of the skin cancers. A recent study, which found over 300,000 new cases in ...
Learn MoreHealthy Aging Month: How Often Should I Get a Skin Exam?

September is Healthy Aging Month, intended to help you and your loved ones stay on top of your health as you age. With that comes a yearly reminder ...
Learn MorePsoriasis Awareness Month: Understand Your Treatment Options
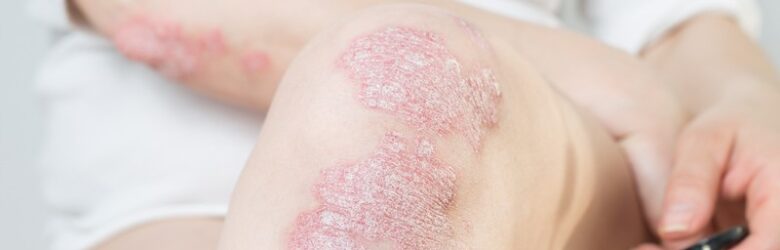
Psoriasis is a chronic skin condition that affects millions worldwide. Though the underlying mechanism is unknown, researchers believe a combination of genetic and environmental factors can lead to psoriasis. If you ...
Learn MoreWhat Type Of Filler Is Best For Treating Smile Lines?
Smile lines and other signs of aging are something that everyone experiences. Many people decide to turn to fillers to help them achieve the youthful look that they are ...
Learn MoreWhat Is a Lip Flip With BOTOX Cosmetic?

We’d all love to have a perfectly plump pout, but if genetics didn’t bless you with one, cosmetic injectables certainly can. While lip filler might be the first ...
Learn More